The COVID-19 Report for Nov 25th, 2020
Maricopa County
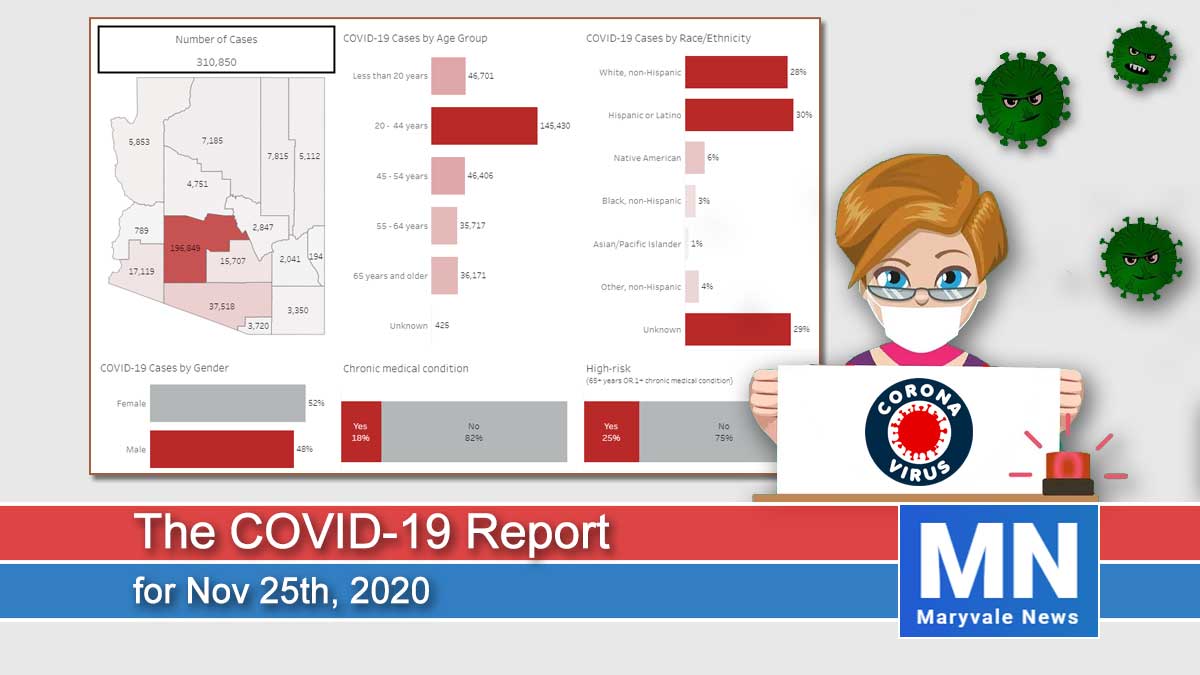
196,849
Total Cases
Maricopa County
2,439
New Cases Today
Maricopa County
5
Deaths Today
Arizona
310,850
Total Cases
Arizona
3,982
New Cases Today
Arizona
9
Deaths Today
USA
12,955,298
Total Cases
USA
175,321
New Cases Today
USA
2,196
Deaths Today
The COVID-19 Pandemic is on the rise in Phoenix and Thanksgiving holiday will probably insure another month of rising numbers. Congress may or may not pass another stimulus bill but people cannot rely on that.
Maricopa County has a program called Take Care that is designed to help keep residents and businesses healthy during the COVID-19 pandemic. County residents can get COVID-19 testing and free flu vaccinations. They can also get rent and utility assistance if they are unable to pay their gas or electric bills or if they are at risk of losing their homes.
The mask mandate for Phoenix remains in effect and face coverings are required in all enclosed public spaces, buses, and trains.
Testing
Phoenix community partners regulary hold free COVID-19 testing sites. Pre-registration is required but it is free to all community members.
As the pandemic increases, testing slows down.
Oxford–AstraZeneca Vaccine
Oxford and AstraZeneca found that the two-dose vaccine had an overall efficacy of 70%. Two dosing regiments were measured, one using two full doses with an average of 62% and one using 1/2 dose followed in two weeks with a full dose had an average of 90%. More testing is needed before approval.
COVID-19 Self-Testing at Home
U.S. Food and Drug Administration issued an emergency use authorization (EUA) for the first COVID-19 diagnostic test for self-testing at home and that provides rapid results.
The Lucira COVID-19 All-In-One Test Kit is a molecular (real-time loop mediated amplification reaction) single-use test that is intended to detect the novel coronavirus SARS-CoV-2 that causes COVID-19.
Moderna COVID-19 Vaccine
Moderna announced that its COVID-19 Vaccine met statistical criteria with a vaccine efficacy of 94.5%. Like the Pfizer Vaccine, it should be remembered that the analysis only included 95 participants with confirmed cases of COVID-19.
Monoclonal Antibody Therapy
The U.S. Food and Drug Administration issued an emergency use authorization for the investigational Monoclonal Antibody Therapy bamlanivimab for the outpatient treatment of mild-to-moderate COVID-19 in adults and pediatric patients. It is currently available in limited quantities now.
The U.S. government has purchased 300,000 doses of bamlanivimab and committed that Americans will have no out-of-pocket costs for the medicine, although healthcare facilities may charge a fee for the product’s administration.
Regeneron monoclonal antibody received EUA from the FDA but is not currently available. It’s authorized for recently diagnosed, mild to moderate COVID-19 in high-risk patients
Pfizer COVID-19 Vaccine
Pfizer and partner BioNTech said that their vaccine against Covid-19 was 90% effective. Although extremely good news it should be taken with a grain of salt. The test period was only over a 7 day period and only included 75 confirmed cases of COVID-19.
It is important to remember that these vaccines have just been invented and we still do not have very much data on how well they work, what the side effects will be, or what the long-term effects will be.
Coronavirus Protection Plan
As the positivity rate in Arizona rises, so does the risk to you and your family. Please protect them and reduce the spread of COVID-19 by:
- Wearing a mask
- Putting at least 6 feet of distance between you and others
- Avoiding all gatherings
- Wash your hands frequently
- Wipe down high-touch surfaces frequently
- Staying home if you can
- Seek medical care if you suspect you have COVID-19