The COVID-19 Report for Nov 20th, 2020

Maricopa County
185,580
Total Cases
Maricopa County
2,403
New Cases Today
Maricopa County
28
Deaths Today
The United States set a new record with 192,240 new cases of COVID-19 in one day. The exponential spread of the Coronavirus continues across the United States at alarming rates with 192,240 new daily COVID-19 cases and 2,065 deaths in a single day. This brings the total number of cases to 12,073,250 since January 2020.
Arizona is also seeing an uncontrollable spread of the Coronavirus and Maryvale is hit especially hard with an 11% positivity rate. We continue to see an increase in case counts, hospitalizations, ICU beds in use, and ventilators in use.
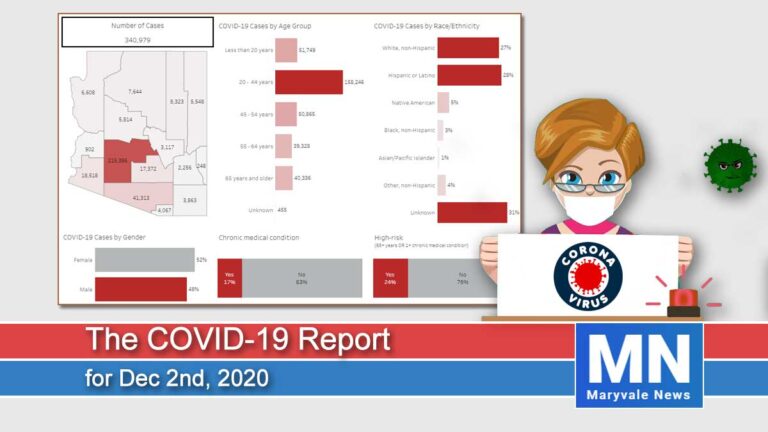
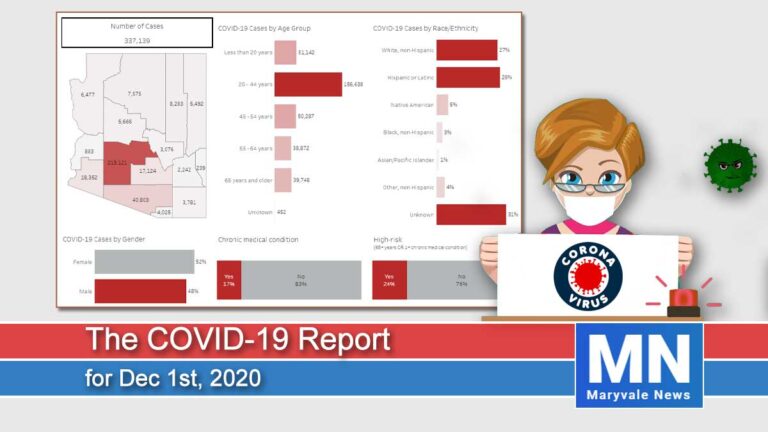
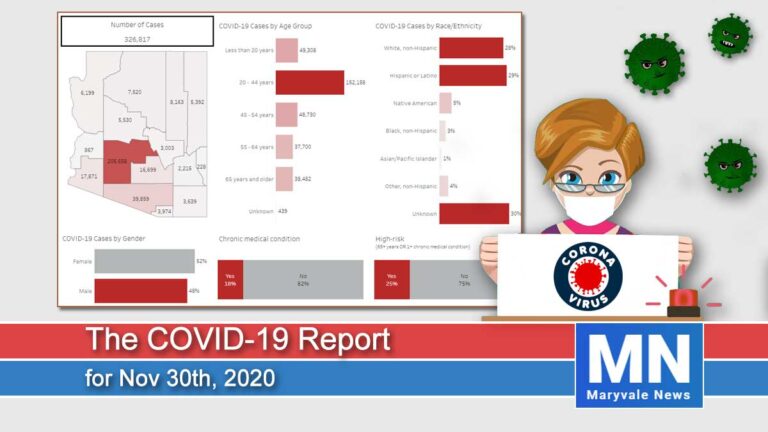
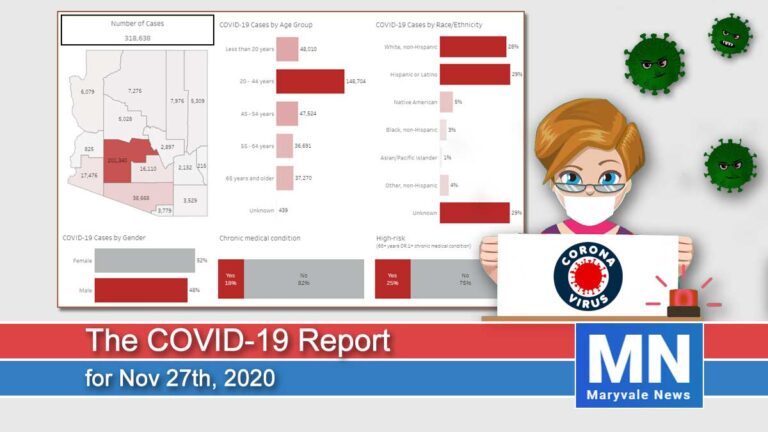
Arizona reported a growth rate of 10.2% in new cases. Today we have had 4,471 new cases and 43 deaths for a total of 291,696 cases since January. The hospitalization rate is at 8% for a total of 24,052 people hospitalized. ICU beds are running out with 431 COVID-19 patients taking up 26% of all ICU beds.
The mask mandate for Phoenix remains in effect and face coverings are required in all enclosed public spaces, buses, and trains.
Testing
The number of people seeking COVID-19 testing is on the rise again. Phoenix community partners are holding free COVID-19 testing sites on select dates. Pre-registration is required. Free to all community members.
As the pandemic increases testing slows down.
COVID-19 Self-Testing at Home
U.S. Food and Drug Administration issued an emergency use authorization (EUA) for the first COVID-19 diagnostic test for self-testing at home and that provides rapid results.
The Lucira COVID-19 All-In-One Test Kit is a molecular (real-time loop mediated amplification reaction) single use test that is intended to detect the novel coronavirus SARS-CoV-2 that causes COVID-19.
Moderna COVID-19 Vaccine
Moderna announced that its COVID-19 Vaccine met statistical criteria with a vaccine efficacy of 94.5%. Like the Pfizer Vaccine, it should be remembered that the analysis only included 95 participants with confirmed cases of COVID-19.
Monoclonal Antibody Therapy
This week, the U.S. Food and Drug Administration issued an emergency use authorization for the investigational Monoclonal Antibody Therapy bamlanivimab for the outpatient treatment of mild-to-moderate COVID-19 in adults and pediatric patients.
The U.S. government has purchased 300,000 doses of bamlanivimab and committed that Americans will have no out-of-pocket costs for the medicine, although healthcare facilities may charge a fee for the product’s administration.
Pfizer COVID-19 Vaccine
Pfizer and partner BioNTech said that their vaccine against Covid-19 was 90% effective. Although extremely good news it should be taken with a grain of salt. The test period was only over a 7 day period and only included 75 confirmed cases of COVID-19. This is not very much data. It could be a fluke or it could be a very powerful vaccine. Without further testing, we cannot know.
Even if it is approved it would be a year before you and I will get it. So do not let your guard down.
Coronavirus Protection Plan
As the positivity rate in Arizona rises, so does the risk to you and your family. Please protect them and reduce the spread of COVID-19 by:
- Wearing a mask
- Putting at least 6 feet of distance between you and others
- Avoiding all gatherings
- Wash your hands frequently
- Wipe down high-touch surfaces frequently
- Staying home if you can
- Seek medical care if you suspect you have COVID-19